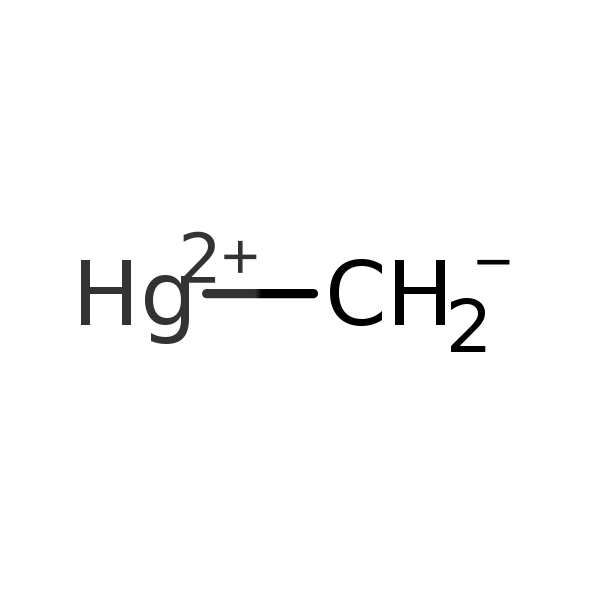
Methylmercury (MeHg)
CASRN 22967-92-6 | DTXSID9024198
- IRIS Summary (PDF) (44 pp, 258 K)
- Status: Methylmercury (MeHg) is in step 1 at this time; see Quick Check.
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF)
(44 pp, 258 K)
Last Updated: 07/27/2001
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Nervous, Developmental | 1 x 10 -4
(High end of BMDL05 range) |
Developmental neuropsychological impairment |
BMDL
5
:
1.5
x 10-3 mg/kg-day |
10 | High |
| Nervous, Developmental | 1 x 10 -4
(Low end of BMDL05 range) |
Developmental neuropsychological impairment |
BMDL
5
:
8.6
x 10-4 mg/kg-day |
10 | High |
Reference Concentration for Inhalation Exposure (RfC) (PDF)
(44 pp, 258 K)
Not assessed under the IRIS Program.
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(44 pp, 258 K)
Last Updated: 05/01/1995
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| C (Possible human carcinogen) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1986) |
- Based on inadequate data in humans and limited evidence of carcinogenicity in animals. Male ICR and B6C3F1 mice exposed to methylmercuric chloride in the diet had an increased incidence of renal adenomas, adenocarcinomas and carcinomas. The tumors were observed at a single site and in a single species and single sex. The renal epithelial cell hyperplasia and tumors were observed only in the presence of profound nephrotoxicity and were suggested to be a consequence of reparative changes in the cells. Several nonpositive cancer bioassays were also reported. Although genotoxicity test data suggest that methylmercury is capable of producing chromosomal and nuclear damage, there are also nonpositive genotoxicity data.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (44 pp, 258 K)
Not assessed under the IRIS Program.
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (44 pp, 258 K)
Not assessed under the IRIS Program.
Program Outlook Details
| Public Assessment Materials | Date |
|---|---|
| Problem Formulation Materials/IRIS Assessment Plan | Apr-2019 |
| Preliminary Assessment Materials/Systematic Review Protocol | May-2020 |
| Public Comment | TBD |
| External Peer Review | TBD |
| Post Final Assessment | TBD |
Note: Any future dates displayed in the table above should be considered estimates and are subject to change. Once the external peer review is complete, estimated dates for release of the final assessment will be published. For the latest information on the status of this assessment, please refer to the IRIS Program Outlook. To access assessment documents, meeting materials or other supporting documents related to this assessment, visit the Chemical Documents tab. For more information about the development process, visit the IRIS Process page.
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
You will need Adobe Reader to view some of the files on this page. See EPA's PDF page to learn more.
Contact Us to ask a question, provide feedback or report a problem.
Quick Check